On science blogs: Immortality
THE IMMORTAL LIFE OF HENRIETTA LACKS GOES ON AND ON. At It Is Not Junk!, evolutionary biologist Michael Eisen argues that discussion about the astonishingly tone-deaf publication of the HeLa cell line's genome a couple weeks ago poses two questions that have been conflated although they are separate.
(An aside: His post is a critique of Rebecca Skloot's op-ed in last Sunday's New York Times. The critique continues in the comments, which feature Skloot's reply and are a fine illustration of a great blogging virtue: the civilized discourse it can encourage when the trolls can be kept away. Is there anyone who does not know that Skloot wrote the best-selling account of HeLa cells, The Immortal Life of Henrietta Lacks?)
The first question, Eisen says, is whether the researchers at the European Molecular Biology Lab in Germany should have gotten permission from Henrietta Lacks's descendants to publish the HeLa cell line's genome. He concludes reluctantly that the answer is yes, as a proxy for Lacks herself, who died of the cervical cancer that HeLa cells were derived from in 1951.
Agreement is pretty much universal on this point. At Tree of Life, evolutionary biologist Jonathan Eisen is more emphatic than his twin, saying he considered sequencing the HeLa cell genome himself in the past but "I decided that it would be unethical, inappropriate, and downright stupid to do this without consent." His is a long post that includes a Storify of tweets about the paper, excerpts from a 2012 Presidential Commission report on genomic privacy, and several links and other commentary.
IS CONSENT ALL RELATIVE? The other issue, Michael Eisen says, is whether a genome can be published even with its possessor's consent if relatives have not also consented. This because publication of your genome will, inevitably, reveal something of the genomes of your parents, siblings, children, etc. His answer is yes, you can consent to publication of your own genome without consulting your relatives.
I can see why on practical grounds alone. Obtaining kin consent would be at best a pain in the ass and often impossible. Also, how far should the quest for permissions be extended ethically? Half-sibs? Aunts, uncles, cousins? Second cousins once removed? Even distant relatives share some potentially identifiable genes.
Although she does not specify how far and wide consent should be sought, at the Mermaid's Tale evolutionary geneticist Anne Buchanan says this is the largest issue. In fact, the EMBL researchers should not only have sought permission to publish, they should have sought permission to do the sequencing in the first place. It is not enough that they have taken the sequence offline temporarily, "the data should be erased from all computers and entirely discarded." Buchanan's position, something of an outlier to be sure, is that Skloot's book was itself an unacceptable invasion of privacy.
Buchanan's is an extreme example, but this is not an entirely silly argument. You can hardly blame your kin if they find publication of your genome unsettling.
It is true that, with rare exceptions, genes are not destiny, a point I and many others have attempted repeatedly to ram home. The fact that I possess a specific gene doesn't mean that my sister does, and even if she does, she will not necessarily develop the same gene-influenced disease that plagues me. But it is also true that genes indicate predispositions, not only to disease but also to behavior — and that the catalog of these genetic revelations is growing every day.
Moreover, scientists have latched on to the study of the many different kinds of epigenetic mechanisms that trigger gene action. So while they continue to investigate nature, they have also begun to investigate nurture in a big and far more precise way. It is not at all farfetched to foresee that eventually they will be able to predict something of a person's future from the genome. Not in my lifetime, and maybe not in yours, but — assuming no calamities intervene — some day.
Thus the question of how much of a say your relatives should have about whether your genome is available for perusal is only going to get more exigent.
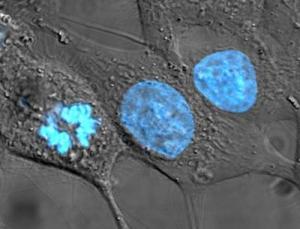
THE HELA GENOME IS NOT REALLY HENRIETTA LACKS'S GENOME This is a point that hardly gets mentioned in the recent public discussion: The HeLa cell line is something of a disputable case for genetic privacy since it is no longer truly Henrietta Lacks's genome.
Publication revealed what has long been suspected, that the genome of HeLa cells is, in the plainspoken words of Nature's Ewan Callaway, "a mess." Most of the chromosomes and many genes have been duplicated, some several times. Chromosomes have also been broken up and reassembled in a different order. The cells have accumulated other mutations in their six-decade journey through thousands of labs. The final kicker: there are several versions of HeLa cells; the EMBL folks sequenced only one, the Kyoto line. Callaway notes:
Without the genome sequence of Lacks’ healthy cells or that of her original tumour, it is difficult to trace the origin of these alterations.
You can get a visual sense of the HeLa genome's disarray if you consult a blog post by Raeka Aiyar, one of the EMBL paper's authors. Aiyar has posted a large graphic, color-coded to display the aberrations, and provides a clear explanation of what it shows. It's worth looking at, but you'll have to go to the site to see it; this site can't display it properly.
HELA CELLS ARE NOT GOOD MODELS FOR NORMAL HUMAN CELLS EITHER. Revelation of what the sequenced HeLa cells contain is not all about genetic privacy. It means that HeLa cells do not really reflect what goes on in normal human cells. It makes you wonder about the validity of more than half a century's life science research and thousands of papers — and also many papers to come, since, Callaway says, apparently researchers will go right on using HeLa cells.
No problem, says Aiyar. Scientists have always known that HeLa cells were not normal human cells and would not yield a normal human genome. This does not "detract from the significant discoveries and technological breakthroughs that HeLa cells have enabled." And yet later in the piece Aiyar wonders whether it's time to reconsider using HeLa cells as a model for human biology. Comparing the standard reference genome generated by the Human Genome Project to HeLa cells, Aiyar says, is like using an Orange County map to navigate downtown Mumbai. Indeed, a major motive for sequencing the HeLa genome at all was to help researchers by defining precisely the differences between the two.
And there is the question of what HeLa cells have done to research on other cell lines that they have invaded. Earlier this month Retraction Watch's Ivan Oransky discussed a paper showing that HeLa cells contaminated the bladder cancer cell line KU7 as long ago as 1984, affecting dozens of papers at a minimum. Oransky argues that those papers should at least carry a notation that the cells used were contaminated by HeLa.
WHAT CAN A PUBLISHED GENOME REVEAL? The Aiyar post is the only blogging I could find by one of the EMBL paper's authors, and it is fascinating for these and other reasons. One is that Aiyar insists that nothing about the Lacks genome can be inferred from the HeLa sequence. That turns out not to be true, as you can learn from the Jonathan Eisen post cited above. EMBL's press release at first made that claim too, but it has been revised and the original author disowned. From the revised EMBL press release:
the genome we sequenced contains a combination of genetic variants originating from the donor’s genome, variants that arose during the tumour’s development, and variants that occurred during the many years of in-lab evolution of the cell line. Comparisons to common genetic variants in human populations today allow one to infer variants likely to have been present in the donor’s genome, although such inferences can only be made with a certain likelihood.
We've had a recent demonstration of how easy it can be to learn people’s identities from genetic data that is supposed to be anonymous. Yaniv Erlich and his colleagues linked "anonymous" genomic data from public databases to particular people by cross-referencing it with other information available online.
This does not mean the public databases should be shut down; they are immensely valuable for research, and it is a condition of genome publication that sequences be deposited in the databases and available to all. “We are in favor of public data sharing, but we need to think about how it could be misused and describe that correctly to people,” Erlich told Technology Review. You can take 15 minutes to watch Erlich explain on YouTube how he and his colleagues used genome databases to identify individuals, one of whom was uber-genomicist Craig Venter
The EMBL researchers took the HeLa sequence offline as the controversy heated up. But it had been downloaded more than a dozen times before it was withdrawn, so it's still out there. And there are chunks of the HeLa genome sequenced by other scientists for other projects; they can be found in various databases. In another post, Oransky notes that the withdrawal puts the paper itself into a kind of limbo. It's still available (open access) as I write, but is now in violation of the requirement that the genome a paper describes must also be freely available.
JOURNALISTS ARE ALSO CLUELESS ABOUT GENOMIC ETHICAL ISSUES, APPARENTLY. At the Columbia Journalism Review's Observatory, Curtis Brainard slammed the reporting on the HeLa genome publication, and he slammed it hard. Journalists, it turned out, were just as blind to the ethical issues surrounding publication as the researchers had been.
One of the oddest things about this event was why it didn't occur to either scientists or reporters to consider whether some sort of permission was required to publish the HeLa genome. It's not as if the odyssey of Henrietta Lacks's cervical cancer cells was unknown. Skloot's book is a careful explanation of how those cells came into universal use without the knowledge of Lacks or her relatives, and without the profit that has accrued to many others, either. The book has been a best-seller, astonishing for a science-based book. Skloot has traveled far and wide on speaking tours and appeared in top-drawer media venues ranging from NPR to Stephen Colbert. (I must look that one up. I wonder if he played it straight, as he sometimes does with topics that even he needs to handle with care.)
In short, this is not an obscure and dusty corner in the history of science. It may even have replaced the infamous Tuskeegee research on untreated syphilis in black men as the most notorious breach of research ethics ever, at least in the US. And it's no accident that both were visited on people who were poor, black, and not consulted or given a choice. I can't help wondering also if that fact helps explain the peculiar blind spots of the EMBL researchers and the media folk who described their work.
So, do you suppose these questions of genetic privacy vs science are coming to a head? It may be fairly easy to say that Henrietta Lacks's descendants should have been consulted before the HeLa (Kyoto) genome was published. It's almost a matter of courtesy, an apology of sorts for the way the family has been treated in the past. But should that apply to all genomes, or be a matter of law?
And what if the family had been consulted, but said "No?"