This student story was published as part of the 2025 NASW Perlman Virtual Mentoring Program organized by the NASW Education Committee, providing science journalism experience for undergraduate and graduate students.
Story by Susan Black
Mentored and edited by Lindsay Brownell
A.I. can design living micro-robots capable of doing (almost) anything.
A.I. hallucinations are usually seen as a dangerous by-product of an overactive, synthetic imagination. From inventing fake scientific publications to generating “phantom” obstacles in self-driving car systems, A.I. hallucinations are ubiquitous and often come with serious consequences. But what if A.I.’s imaginative power could be harnessed to drive innovation instead?
An early adopter of A.I.-driven scientific research is Josh Bongard, a scientist at the University of Vermont. Bongard and his team of researchers are best known for their invention of xenobots: living micro-robots made from clusters of cells. They created these robots to explore A.I.’s ability to design living beings with specific capabilities, as well as what this novel, pre-programmed material could do. While other labs have built robots from living cells before, xenobots are unique in that they owe their existence to generative A.I.
Bongard leverages deep learning and evolutionary algorithms to propose various body plans for his bots, which are tailored to fit specific tasks. When asked about the oversight of these algorithms, Bongard said that they operate with minimal input and few constraints. The “[proposed body plans are] alien in the sense that [they’re] not designed in the way that a human would design things.” This design strategy, free of human intervention, enables the A.I. to explore a broad range of possibilities, some of which are beyond our conception.
According to Bongard, both humans and A.I.-powered machines can “design things out of cells, but [the approaches they take are] very different.” In natural organisms, body plans are encoded in genes and constructed through intricate biochemical signaling pathways. Xenobots, however, are different: their body plans are entirely imagined by A.I., not genes. Bongard likened their designs to “alien artifacts” that are completely “immune to human interpretation.” As a result, Bongard and his colleagues don’t fully understand why these body plans work, only that they do. As he puts it, “the A.I. is doing the heavy lifting” during the early stages of xenobot synthesis.
Bongard and his team first test the body shape configurations imagined by the A.I. in silico to identify the best performing bot shapes. To bring the A.I.’s designs to life, clusters of embryonic frog cells are meticulously sculpted by hand in vitro using microsurgery tools. According to Bongard, it can take about four hours to make one poppy seed-sized xenobot.
Next, the researchers evaluate a bot’s ability to perform specific tasks. In the first iteration in 2020, Bongard and his team created xenobots that could “walk” using strategically placed contractile heart muscle cells. These bots traversed their environment with surprising ease, pushing against the surface of their dish to gain traction.
Beyond movement, certain bot designs displayed other unexpected properties: They could self-heal, self-replicate, and self-destruct. After incurring damage, xenobots will quickly close any wounds and return to their original spherical shape. Along with this ability to heal, the micro-bots can also self-replicate. Rather than our traditional definition of “reproduction” as a single cell dividing into two genetically identical daughter cells, xenobots perform “kinematic replication.” When xenobots are placed in a dish with loose stem cells, they can gather the cells into clusters that then fuse together to form new, functional, individual bots. And after approximately a week in culture, the cells comprising an individual bot will dissociate, and the xenobot will degrade, ending their unusual life cycle.
Bongard was quite excited and surprised by these behaviors. He encourages others to “imagine building machines and materials from parts that are smart, that can heal and replicate themselves.” Using living material with emergent properties, as Bongard and colleagues have done, could revolutionize the future of engineering. In other words, “it’s not just a [new] technology, it’s a [new] way of doing technology,” he said.
Although these xenobots are impressive and intriguing in their own right, they also have wide ranging applications across multiple industries. From aggregating and degrading microplastics in our oceans to clearing plaque from blood vessels, living micro-bots could fill critical gaps in the market and be engineered to perform a vast array of essential tasks. They could even be used for future problems that have not yet materialized. In essence, their usefulness spans far beyond what we humans might envision ourselves, bounded only by what A.I. can imagine.
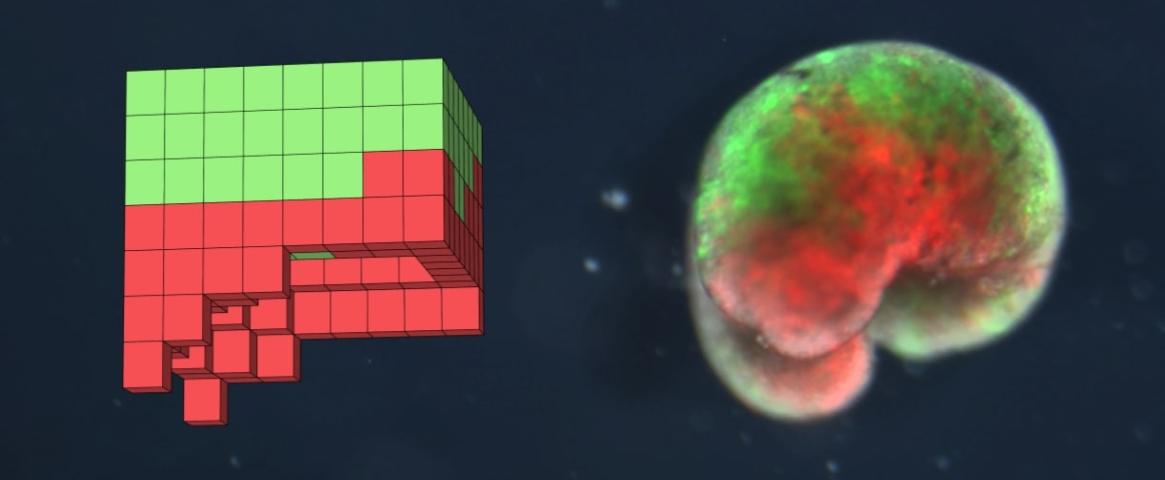
Top image : A.I. designed Xenobot. Color is used to distinguish between different cell types. Red indicates frog heart muscle and green represents frog skin cells. On the left is the computational rendering of the design. On the right is a living Xenobot. Image credit: Josh Bongard and his team.
Susie Black is a graduate student at Boston University studying gene regulation in the laboratory of Zeba Wunderlich. She completed her undergraduate degree in biology at Wellesley College. She then worked at Boston Children’s Hospital in a hematology oncology lab studying germline predisposition to leukemia. In her downtime, she loves to run along the Muddy River and read mystery books. Contact: linkedin.com/in/susie-black-4097891a0, susieb12@bu.edu.
Mentor: Lindsay Brownell graduated from MIT’s Graduate Program in Science Writing in 2014 and has been a science writer at a variety of organizations in both academia and industry for over a decade. She earned a dual B.S/B.A. in biology and English, respectively, from Davidson College. In addition to writing, she is a volunteer editor for Science Homecoming, a co-founder of nonprofit Ampl, and a 305 Fitness instructor.
The NASW Perlman Virtual Mentoring program is named for longtime science writer and past NASW President David Perlman. Dave, who died in 2020 at the age of 101 only three years after his retirement from the San Francisco Chronicle, was a mentor to countless members of the science writing community and always made time for kind and supportive words, especially for early career writers.
You can contact the NASW Education Committee at education@nasw.org. Thank you to the many NASW member volunteers who lead our #SciWriStudent programming year after year.
Founded in 1934 with a mission to fight for the free flow of science news, NASW is an organization of ~2,600 professional journalists, authors, editors, producers, public information officers, students and people who write and produce material intended to inform the public about science, health, engineering, and technology. To learn more, visit www.nasw.org.