This student story was published as part of the 2025 NASW Perlman Virtual Mentoring Program organized by the NASW Education Committee, providing science journalism experience for undergraduate and graduate students.
Story by Katelyn L. Richard
Mentored and edited by Lesley Earl
We emit chemicals from our breath and skin that tell a story. These chemical emissions change whether we are in a lunch meeting, watching a scary movie, at the gym, or on a date, for example. As these natural chemicals from our skin interact with the air, we generate and are surrounded by a chemical “field.” Scientists have recently discovered that lotions and perfumes disrupt this field, impacting not just the chemicals emitted by our skin, but also how those chemicals react with what’s in the air around us. And when we’re indoors, where there’s less air flow than outside, these products could play a big role in the quality and chemical content of the air we breathe.
We spend up to 90% of our time indoors. While there has been a lot of research on indoor air quality, most studies “had been discussing emission from furnishings or curtains or clothing or cooking or cleaning,” said Jonathan Williams, a group leader at the Max Planck Institute for Chemistry in Germany. “But people had been ignoring the big smelly thing in the room, which is the human being.”
As a practical example, Williams explained that when you sit down on the sofa, you are not just breathing in the chemical from the sofa but also the chemicals formed by interactions between us and the sofa. To take this analogy a step further, once you add a human to the sofa, you must then consider how the personal care products they have on react with their skin and the sofa.
Ozone is an important air pollutant when it comes to reactions with sofas, skin, personal care products and other items indoors, because ozone is responsible for indoor oxidation — a chemical process that can form harmful byproducts. Scientists recently discovered that ozone interacts with the chemicals from our skin to form the hydroxyl radical (OH), a tiny molecule in the air that exists in a field around each of us. Researchers often refer to this as the “human OH field.”
OH is even more efficient at oxidation than ozone, since it reacts more quickly with nearly all chemicals indoors. But the discovery of the human OH field led researchers to a host of new questions: What happens to this OH field if we put something on our skin? And what happens to the air around us as the OH field increases or decreases? Do changes in the air have any impact on our health?
To address these questions, Williams and Nora Zannoni, a researcher in his lab, collaborated with researchers from the Technical University of Denmark. Together, they assembled a group of volunteers who spritzed themselves with perfume or lathered on lotion, and then sat in a small stainless steel room so the scientists could measure the chemicals their skin emitted.
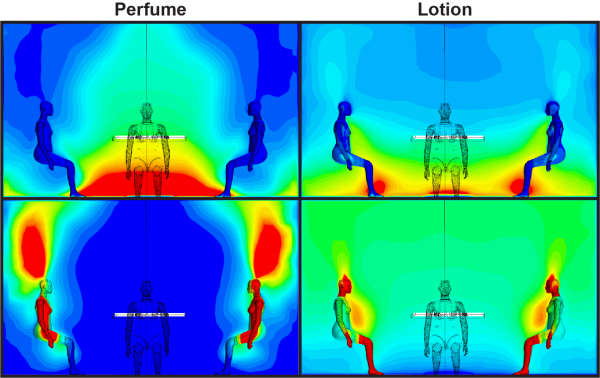
Images based on modeling and analysis performed by Pennsylvania State University and University of California, Irvine researchers. Top panels: OH concentrations after perfume and lotion applications. Bottom left: Ethanol (perfume product) concentration after perfume application. Bottom right: Phenoxyethanol (lotion product) concentration after lotion application. For all images, dark blue represents lowest concentration and red represents highest concentration. Credit: Nora ZannoniThe researchers concluded that perfume and lotion both decreased the human OH field. Lotion, due to its thick, absorbent nature, decreased the human OH field slowly over a longer period, whereas perfume caused an extreme, yet momentary, decrease in the OH field.
Depletion of the human OH field limits oxidation and thus the production of chemicals that are known to negatively impact human health — but the story does not end there. We don’t yet know how toxic these chemical byproducts of personal care products are in indoor spaces, explained Zannoni. “So, it’s hard to say … if the human oxidation field is protecting us or not,” or if personal care products make indoor air quality better or worse.
Top image: Humans have a chemical field around them that is altered by application of personal care products. Credit: Katelyn Richard
Katelyn Richard is a Ph.D. candidate studying atmospheric chemistry at Colorado State University. As a part of her research, Katelyn measures outdoor air to identify traces of human-made chemicals such as cleaning solutions and personal care products. You can reach her on LinkedIn or via email at katelyn.richard@colostate.edu.The NASW Perlman Virtual Mentoring program is named for longtime science writer and past NASW President David Perlman. Dave, who died in 2020 at the age of 101 only three years after his retirement from the San Francisco Chronicle, was a mentor to countless members of the science writing community and always made time for kind and supportive words, especially for early career writers.
You can contact the NASW Education Committee at education@nasw.org. Thank you to the many NASW member volunteers who lead our #SciWriStudent programming year after year.
Founded in 1934 with a mission to fight for the free flow of science news, NASW is an organization of ~2,600 professional journalists, authors, editors, producers, public information officers, students and people who write and produce material intended to inform the public about science, health, engineering, and technology. To learn more, visit www.nasw.org.