By Sophia Schmidt
BOSTON — While scientists have not yet made changes to human reproductive cells that can be passed down through generations, the most recent breakthrough in gene editing technology—CRISPR-Cas9—has brought us to the brink of this possibility. With technological innovations hurtling ahead, ethics and legal groups say we need to align genetic modification science with a moral compass, and consider the perspectives of the general populace.
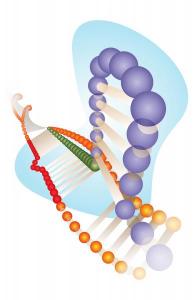
CRISPR-Cas9 allows scientists to cheaply and easily cut DNA at a specific location. Once the DNA strand is cut, proteins within the cell begin to repair it. During this repair process, mutations often occur that change the DNA sequence, sometimes with the help of scientists.
Gene editing in reproductive cells, called germline editing, may pose a greater ethical risk than gene therapy in non-reproductive, or somatic, cells. That’s because germline edits may carry on to future generations and could alter the human gene pool irreversibly. For example, some envision a loss of diversity if it becomes common practice to “correct” appearance or varying levels of cognitive and athletic ability.
A new report from the National Academy of Sciences and the National Academy of Medicine supports limited germline editing as last-resort prevention for serious genetic diseases and disabilities such as cystic fibrosis or muscular dystrophy. Germline editing should never be used for pure trait enhancement—to build, for example, children that are smarter, stronger, or look a certain way, the report said.
But how should the line between acceptable and unacceptable uses of gene editing technology be determined? And how should it be enforced?
In the United States, human genetic modification is within the purview of the Food and Drug Administration. But the FDA can only legally consider safety and efficacy in decisions regarding regulation of medical technologies. Ethical issues cannot be considered.
“There are reasons why we don’t give FDA this authority, perhaps,” said Gary Merchant, professor at the Sandra Day O’Connor College of Law at Arizona State University. “Beyond ‘does this cause cancer?’ or ‘does this work?’ we don’t really have a common consensus on what’s ... good or bad in the ethical realm. These are much more controversial concepts ... harder to define, harder to quantify.”
Government agencies such as the FDA are generally staffed with scientists, career administrators, lawyers, politicians, and business people. One potential solution to dealing with the ethics issues raised by gene editing would be hiring ethicists and religious experts to serve in health-related agencies, Merchant said. This idea could conflict with the First Amendment’s separation of church and state, however.
Another potential solution is an expansion of the FDA’s definitions of safety and efficacy to address broader social risks. Alternately, laws could be changed to give agencies the “express ability to consider ethical [and] social concerns,” Merchant said. Perhaps an ethical impact statement could be required of health-related agencies, akin to the currently needed environmental, economic impact statements for major initiatives.
Instead of a government agency, Congress may be the best equipped to deal with genetic modification regulation, as it does not have limitations on ethical considerations, Merchant said. “As long as it’s constitutional ... they can consider any ethical, social thing they want. The question is, should we give all these decisions to Congress?”
And it’s not just lawmakers who would need to have a say. The process must also include the voice of the general public, Merchant said.
“The big challenge is, how do you incorporate [public engagement] into the decision making process?”
Sophia Schmidt is a senior at Williams College studying biology and environmental policy. Reach her at ses5@williams.edu or tweet @SchmidtSophia8
